Cancer could be detected 3 years before symptoms appear with a simple blood test; new study reveals
A groundbreaking study by Johns Hopkins University researchers reveals that a simple blood test could potentially detect cancer years before symptoms manifest. This innovation offers a new horizon for early diagnosis and preventative measures.
Early detection is crucial in improving survival rates and broadening treatment options for cancer patients. The study, featured in Cancer Discovery, suggests that this new blood test could be a significant step forward.
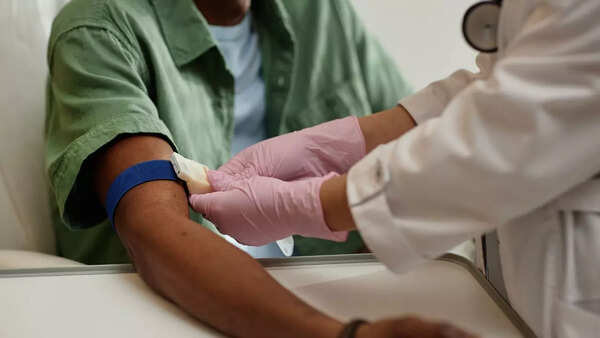
Early Intervention: A Key to Successful Treatment
"Three years earlier provides time for intervention," explains researcher Yuxuan Wang from Johns Hopkins. Identifying tumors in their early stages can make them more receptive to treatment. This head start could be the determining factor between a curable condition and a life-threatening one, particularly in aggressive forms of cancer.
The study focuses on circulating tumor DNA (ctDNA), genetic material that tumors release into the bloodstream. While these traces are extremely small and challenging to detect, especially in the early stages, identifying them could revolutionize cancer detection.
The Science Behind the Blood Test
The scientists employed sophisticated algorithms to analyze blood samples for specific modifications in DNA patterns indicative of tumors. This technique is the foundation of a Multi-Cancer Early Detection (MCED) test, designed to identify cancer-specific genetic changes in the blood.

The research team examined blood samples from 52 individuals:
- 26 who were diagnosed with cancer within six months of sample collection.
- 26 who remained cancer-free.
The MCED test successfully flagged eight cancer cases, demonstrating a 31% detection rate before any formal diagnosis or visible symptoms.
Revealing Insights: Testing the Method
The study's most remarkable finding was the analysis of older blood samples from some participants. Among the eight individuals detected by the MCED test, six had blood samples available from 3.1 to 3.5 years prior to their diagnosis. Astonishingly, cancer signals were detected in four of those six samples.
The presence of ctDNA, even at levels significantly lower than the current test threshold, indicates that tumors begin shedding DNA into the blood long before symptoms appear. Improving the sensitivity of these tests is crucial for capturing these early signs.
Dr. Bert Vogelstein, a senior cancer researcher, emphasizes, "This study shows the promise of MCED tests in detecting cancers very early. But it also sets the benchmark sensitivities required for these tests to succeed."
Navigating the Path Forward
The transition from laboratory research to clinical application requires rigorous clinical trials to validate the reliability and safety of blood-based cancer screening tests. Regulatory approvals are also necessary before these tests can be integrated into routine medical practice.

Dr. Nickolas Papadopoulos from the Ludwig Centre highlights the need to determine the appropriate clinical follow-up procedures after a positive test result, including further scans, biopsies, and potential preventative treatments.
Despite these challenges, this research signifies a significant step forward in cancer diagnostics, offering the potential for improved survival rates through early detection and treatment. This breakthrough could revolutionize how cancer is screened and treated.
Newer articles
Older articles
-
 How do graphic designers convert JPG to PDF (Portable Document Format)?
How do graphic designers convert JPG to PDF (Portable Document Format)?
-
 Priyanka Chopra reveals daughter Malti Marie has started school in New York: 'Her schedule is even crazier than mine'
Priyanka Chopra reveals daughter Malti Marie has started school in New York: 'Her schedule is even crazier than mine'
-
 Mahbub Anam replaces Faruque Ahmed as new BPL chairman
Mahbub Anam replaces Faruque Ahmed as new BPL chairman
-
 This new AI tool can help you book train tickets, get refunds and check details on IRCTC website and app
This new AI tool can help you book train tickets, get refunds and check details on IRCTC website and app
-
 Twitter bans over 5 lakh accounts in India, here's why
Twitter bans over 5 lakh accounts in India, here's why
-
 Karisma Kapoor's ex-husband Sunjay Kapur's alleged last rescue clip shows him getting CPR on the ground - WATCH VIDEO
Karisma Kapoor's ex-husband Sunjay Kapur's alleged last rescue clip shows him getting CPR on the ground - WATCH VIDEO
-
 Mom-to-be Malvika Raaj shares dreamy pictures from her 'Godh Bharai' ceremony with Guruji Satsang
Mom-to-be Malvika Raaj shares dreamy pictures from her 'Godh Bharai' ceremony with Guruji Satsang
-
 Salman Khan reveals struggle with AVM and brain aneurysm on Kapil Sharma Show: Know what AVM is, its causes, and why it's so serious
Salman Khan reveals struggle with AVM and brain aneurysm on Kapil Sharma Show: Know what AVM is, its causes, and why it's so serious
-
 Microsoft plans to take on iPhone and Android smartphones with this new device
Microsoft plans to take on iPhone and Android smartphones with this new device
-
 Priya Sachdev looks devastated as she breaks down in tears at husband Sunjay Kapur's funeral in New Delhi - See photos
Priya Sachdev looks devastated as she breaks down in tears at husband Sunjay Kapur's funeral in New Delhi - See photos